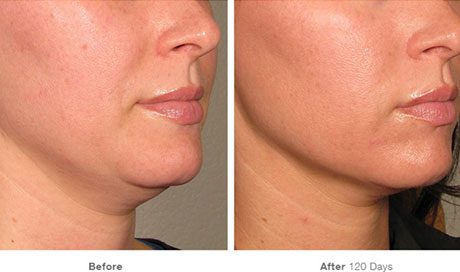
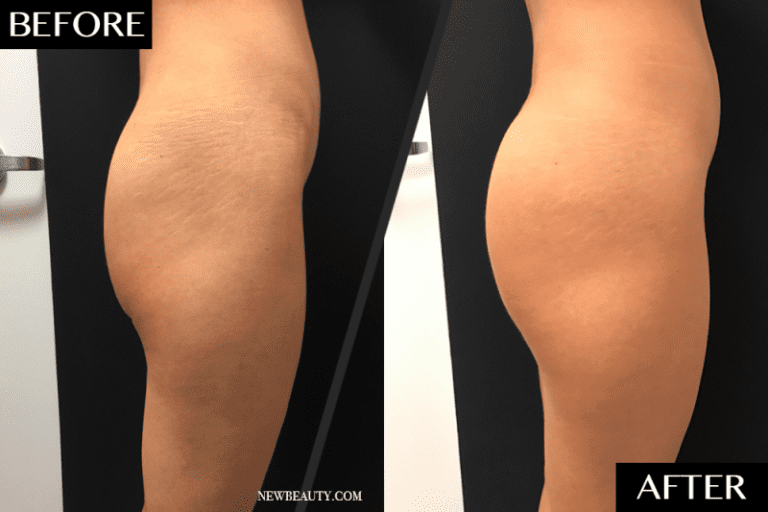
The quest for safe and effective weight loss solutions is a never-ending journey for many. Semaglutide, a drug initially developed for type 2 diabetes treatment, has recently gained attention for its promising weight loss effects. While the potential of this medication is exciting, it’s essential to understand the FDA’s stance on semaglutide and the importance of obtaining it safely. This blog post will guide you through the ins and outs of semaglutide, FDA regulations, safety concerns, and how to make informed decisions for weight loss.
We will delve into the different options available in terms of drug preparations, their mechanisms of action, and the FDA’s most recent regulations on peptides and compounding. By the end, you’ll have the knowledge and confidence to navigate the world of semaglutide for weight loss safely and effectively.
Key Takeaways
- Semaglutide is a FDA approved weight loss drug administered through weekly subcutaneous injections.
- FDA regulations on peptides and compounding have an impact on semaglutide’s status, so patients should be aware of the risks associated with using it for weight loss.
- Consult a healthcare professional and obtain semaglutide from regulated sources to ensure safety when considering this prescription medication as part of a weight management plan.
Introduction to Semaglutide for Weight Loss
Semaglutide is a GLP-1 receptor agonist, which mimics the GLP-1 hormone and is indicated for type 2 diabetes treatment, blood sugar control, and weight loss. Semaglutide belongs to a class of medications known for their weight loss potential. Brands like Ozempic and Wegovy have gained popularity for this reason. Wegovy is an FDA-approved once-weekly injection for chronic weight management, while Ozempic was specifically formulated for type 2 diabetes patients. Most recently Zepbound by Eli Lilly has been FDA approved for weight loss and shows great hope for the battle against obesity.
Ozempic functions by replicating a naturally occurring hormone that signals the brain of satiety and slows down digestion, similar to the effect of bariatric surgery. It is often used in conjunction with lifestyle changes, such as diet and exercise, for weight loss. The recommended dosage of semaglutide for weight loss is 2.4 milligrams, administered weekly via subcutaneous self-injections.
The FDA approved weight loss drug health care providers sometimes prescribe is Wegovy. Gastrointestinal disturbances are the most frequently reported adverse reactions among individuals commencing semaglutide therapy, therefore a thorough history should be discussed with your health care provider prior to initiating therapy. Risk vs benefit ratio should always be considered whether starting an FDA approved drug or a compounded medication.
FDA’s Stance on Peptides and Impact on Semaglutide
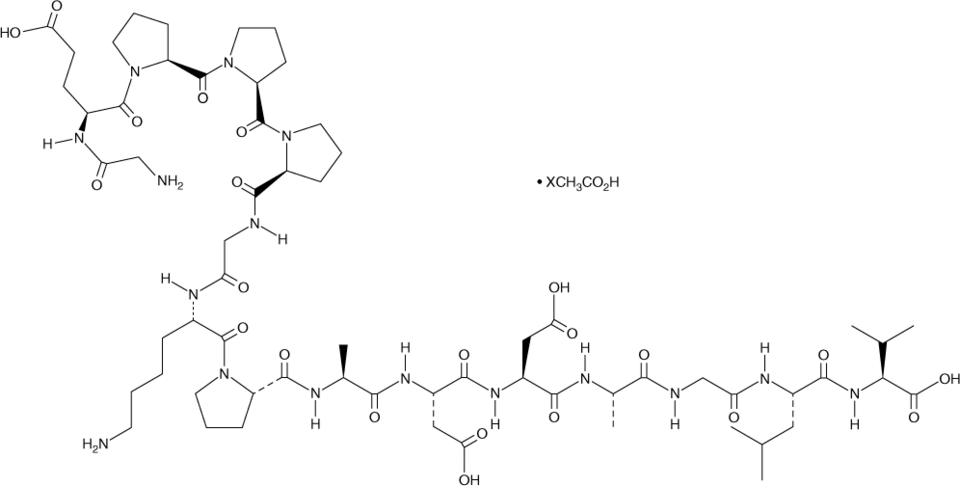
The FDA regulates peptides as drugs, not biologics, under the Federal Food, Drug, and Cosmetic Act. However, the recent reclassification of certain peptides as biologics may have an effect on semaglutide’s compounding status. This reclassification could potentially impact the use of bulk drug substances in its formulation.
The FDA cautions against utilizing “generic” Ozempic and Wegovy and states that there are no approved generic versions of these medications. Patients should be aware that products advertised as ‘semaglutide’ may not contain the same active ingredient as semaglutide products, approved by FDA. They may, instead, include salt formulations. Semaglutide sodium and semaglutide acetate have not been proven to be safe and effectiveness has not been established.
For patients considering using this drug for weight loss, it’s imperative to grasp the FDA’s regulations on peptides and semaglutide. This knowledge will help patients navigate the complex world of semaglutide safely and effectively.
Understanding FDA’s Compounding Regulations and Semaglutide
Compounding semaglutide is feasible under certain conditions, such as in the case of shortages under the Federal Food, Drug, and Cosmetic Act. However, the FDA has cautioned against utilizing compounded versions of semaglutide due to potential safety and efficacy issues, as they may not have undergone the same rigorous FDA approval process as the original drug.
Distributing unapproved compounded products, such as semaglutide, can be a costly misstep, and may incur fines and potential legal action. One pharmacy was ordered to pay a fine of over $1.7 million for distributing the first drug of this kind without proper approval. Additionally, the owner received a significant fine and three years of probation.
Comprehending the FDA’s compounding regulations and the potential risks associated with compounded semaglutide equips patients to make more informed decisions about obtaining and using this weight loss medication. The NF monograph articulates the quality expectations for any medication, not just the active ingredients.
Safety Concerns with Non-Approved Semaglutide
The FDA has issued warning letters to cease the circulation of unlawfully marketed semaglutide products that have not received FDA approval. Non-approved semaglutide products may:
- be counterfeit
- contain incorrect active ingredients
- have inadequate amounts of active ingredient
- have excessive amounts of active ingredient
- have no active ingredient
- contain other potentially harmful ingredients.
Using non-approved semaglutide products may have potential impacts on your health overall. These products may not contain the same active ingredient as FDA-approved semaglutide, which means they may not have gone through the same rigorous testing and quality control measures. Compounded semaglutide products, in particular, can be cause for concern as they may be formulated with different ingredients that can lead to unexpected side effects.
Furthermore, substituting FDA-approved medications with non-approved semaglutide products can increase the risk of severe stomach problems and other adverse effects. To mitigate these risks, patients should go through licensed health care providers and avoid purchasing from unregulated sources.
Health benefits of Semaglutide
One thing is for certain, the effectiveness of this medication for obesity is causing an unprecedented demand. Along with lifestyle changes including exercise, many patients have left the obese category. Studies have shown the health benefits to include a decrease in the risk of stroke and heart attack. Along with diet and exercise, diabetes drugs like Semaglutide could make obesity a thing of the past.
Guidance on Obtaining Semaglutide Safely
Patients should consult their primary care physician and discuss their weight loss goals and concerns to safely obtain semaglutide. Although many patients seeking drugs in this category may be obese, many overweight patients with risk factors such as family history of diabetes may also be good candidates. The FDA guidelines stipulate that patients should only obtain prescription weight loss drugs like semaglutide with a prescription from a licensed healthcare provider.
Patients are advised to procure drugs containing semaglutide with a prescription from a certified health care provider and acquire medications from state-licensed pharmacies or outsourcing facilities registered with the Food and Drug Administration (FDA).
Consulting with a primary care provider is the best way to gain more information about semaglutide and other weight loss medications for individuals looking to lose weight. Patients can ensure they are using a safe and effective medication for their weight loss journey when they follow the proper channels for obtaining semaglutide.
Risks of Online Semaglutide Purchases
Purchasing semaglutide from unregulated sources online may result in exposure to counterfeit or hazardous compounded products, which could present considerable health risks. Obtaining medicine from unregulated, unlicensed sources online can potentially expose patients to products that have not been evaluated or approved adequately, or do not adhere to quality standards for active ingredients.
The potential health implications of using counterfeit semaglutide for weight loss could be severe, including hypoglycemic shock, coma or even death. Additionally, counterfeit pens may contain insulin instead of semaglutide which again could have significant negative effects.
To avoid these risks, patients should only purchase semaglutide from regulated sources with a valid prescription from a healthcare provider. This will help ensure that they are receiving a safe and effective medication to support their weight loss goals.
How to Report Semaglutide-related Issues to the FDA
The FDA encourages reporting of adverse events and quality issues related to semaglutide through their MedWatch program. Patients can submit adverse events and quality issues related to semaglutide through the FDA’s MedWatch program via the MedWatch Online Reporting Form, by calling 1-800-FDA-1088, or by downloading a reporting form and returning it to the FDA.
Patients can contribute to the FDA’s ongoing efforts to ensure the safety and efficacy of this medication when they report semaglutide-related issues. This collaborative effort between patients, healthcare providers, and the FDA plays a critical role in safeguarding public health.
New Regulations on Peptides
The Latest regulations regarding Peptides that have many consumers concerned are actually not aimed at weight loss medications. Peptides such as BPC-157 (Body Protection Compound), Ipamorelin, Kisspeptin, and Sarcotropin are what have been targeted. Many health care professionals have been up in arms about this bulk drug substances list that has been under attack. Rightly so, many health care professionals have taken to social media as they have treated thousands of american adults with these peptides and have seen only benefits and way less harm compared to approved drugs.
Peptides have been valuable to many american adults for a wide range of health benefits and peptides such as Sarcotropin and Ipamorelin will be greatly missed. These peptides are not technically illegal at the time of writing this article however the FDA has announced they may start coming after any compounding pharmacies found to be compounding these substances and therefore most human drug compounding pharmacies have begun clearing their shelves of peptides.
Conclusion: Making Informed Decisions for Weight Loss
For those considering the options discussed for weight loss, it’s essential to make informed decisions by understanding FDA regulations, safety concerns, and the appropriate methods for obtaining the medication. Consulting with a healthcare professional, such as the experts at DHH Med Spa, and following their guidance is crucial to ensure a safe and effective weight loss journey.
Megan Davies FNP-BC, CANS has always had a passion for helping her patients reach their weight loss goals. Originally in 2015 when DHH Med Spa was started Megan opened the clinic under the name Desert Holistic Health. She had previously worked for an integrated primary care office. At the office she worked with a Naturopath, Chiropractor and Osteopathic Doctor. She loved discussing lifestyle modification with her patients.
Megan has a few stories she could share with you about patients who got their lives back and had told her they had wished they had found her years ago. As life got busier and more patients referred their friends for aesthetic services her shift changed. With the introduction of GLP-1 receptor agonists on the market she loves that she is now able to offer an option that has such powerful results.
Summary
In conclusion, semaglutide holds great promise as a weight loss solution, but understanding FDA regulations, safety concerns, and proper channels for obtaining the medication is essential. By consulting a healthcare professional, obtaining medication from regulated sources, and adhering to FDA guidelines, patients can achieve their weight loss goals safely and effectively. Don’t be afraid to ask your health care provider to see the bottle- semaglutide sodium is what the FDA is warning against in the world of compounding.
Frequently Asked Questions
Is semaglutide approved by the FDA?
Semaglutide, also known as Wegovy, is an FDA-approved anti-obesity medication that can be used in adults and pediatric patients as an adjunct to a reduced calorie diet and increased physical activity for chronic weight management. It was approved in 2021 by the U.S. Food and Drug Administration (FDA).
What does semaglutide do to your body?
Semaglutide works by increasing insulin levels in the body, reducing the amount of sugar released into the blood and slowing digestion – all of which can help with blood sugar management (diabetes) and weight loss.
I heard there is a new ban on Peptides, does this mean Semaglutide is going away?
The new ban is on specific peptides such as BPC-157. In our Scottsdale office this has been a peptide we have found incredibly helpful for stomach issues related to Semaglutide. Unfortunately due to the ban, this peptide along with Sarcotropin, which we carried will no longer be available for purchase after our stock is depleted.